
If not, pick a new temperature and repeat KA(Tnext) KA(Tprev)(xi)previRelative volatility volatility KA yAxA KA(T)ĪB KAKByAxAyBxBstrong function of temperature not a strong function of temperature often assumed independent relative volatility for a binary system, substitute and rearrange: yAĪBxA1+(AB 1)xAyB 1yAxB 1xABubble point calculation using relative volatility Kref1 Pick a temperature T and find the corresponding Ki(T) values for each component 2.
DEPRIESTER CHART ISOBUTANE HOW TO
If not, pick a new temperature, repeat How to pick a temperature? How to pick the next temperature? To achieve rapid convergence: Initial guess:(weighted average of boiling points of pure components) T ziTii(Ki 1)KA(Tnext) KA(Tprev)(yi)previNext guess: pick a reference component (A) find Tnext using DePriester Chart Dew point calculation for multi-component mixtures Trial-and-error method Given the composition of a superheated vapor and PTOTAL,find Tdp and (xi)dp VLE: mole balance: xi yiKixii1.0Algorithm: 1.
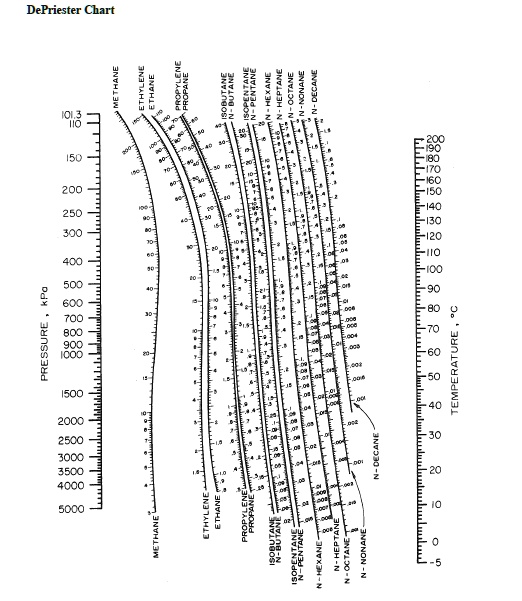
PA0(T)PTOTALactivity coefficient fugacity coefficient vapor pressure Bubble point calculation for multi-component mixtures Trial-and-error method Given the composition of a subcooled liquid and PTOTAL,find Tbp and (yi)bp VLE: mole balance: yi Kixiyii1.0Algorithm: 1. At 2000 kPa, what is the boiling point of ethane? volatility At 15 C, what is the saturated vapor pressure of isobutane?At 0 C and500 kPa, what is the volatility of n-hexane?Using data from vapor pressure tables Raoults Lawideal liquid: non-ideal liquid: Daltons Lawideal gas: non-ideal gas: PA xAPA0(T)PA AxAPA0(T)yA PAPTOTALyA PA Wankat(ISBN: 0131382276) Copyright 2012 Pearson Education, Inc. Prog.,85, April 1978 copyright 1978, AIChE reproduced by permission of the American Institute ofChemical Engineers) From Separation Process Engineering, Third Edition by Phillip C. Useful definitions Boiling/bubble point Tbp: temperature at which the average liquid molecule has just enough kinetic energy to escape from the surface of the liquid into the gas phase Recall that kinetic energy follows a Boltzmann distribution, so molecules with higher than average kinetic energy can still escape from the surface at T 1 prefers vapor phase K 1 T* P* K < 1 Figure 2-11Modified DePriester chart (in S.I. What is the boiling range of this mixture? Boiling point, dew point, bubble point Pure liquids have a boiling point mixtures have a boiling range, delimited by their bubble point and dew point. Consider a superheated binary vapor that is 40 mol% ethanol.What is its dew point?What is the composition of the first drop? 3. Consider a sub-cooled binary liquid that is 40 mol% ethanol.What is its bubble point?What is the composition of the first bubble? 2.

Figure 2-3Temperature-composition diagram for ethanol-water bubble point dew point 圎,initial yE,initial boilingrange 1. yA A subcooled liquid feed of composition zA, heated to temperature TA, will separate spontaneously into 2 phases, of composition xA and yA TA xA yA zA From Separation Process Engineering, Third Edition by Phillip C.

Figure 2-3Temperature-composition diagram for ethanol-water superheated vapor subcooled liquid 2-phaseregion saturated vapor line saturated liquid line 2 graphs in one: T vs. Goal 1: Design a flash drum How big should the drum be? What height should the nozzle be? What T and P should the drum be? What T and P should the feed be? Vapor-liquid equilibrium (VLE) Consider a binary (i.e., 2-component) system with 2-phases: Tvap, Pvap yA, yB Tliq, Pliq xA, xB What do we know? yA + yB = 1 xA + xB = 1 yA xAĪt equilibrium:Tvap = Tliq Pvap = Pliq Gibbs Phase Rule: degrees of freedom = # components (C) - # phases (P) + 2 For a binary, 2-phase system: 2 2 + 2 = 2 We can specify only 2 intensive variables (all others are fixed, by VLE) Specify P and T From Separation Process Engineering, Third Edition by Phillip C.


 0 kommentar(er)
0 kommentar(er)
